WHAT WE ARE MADE UP OF?
Understanding the structure of an atom
“Protons give an atom its identity, electrons its personality.” ― Bill Bryson
Previously we have seen some basic things about the particles present in the atom. This segment will mainly focus on the detailed structure of an atom, the rules governing them, and the parameters to locate the three fundamental particles of an atom. So this segment needs little attention to understand the concept.
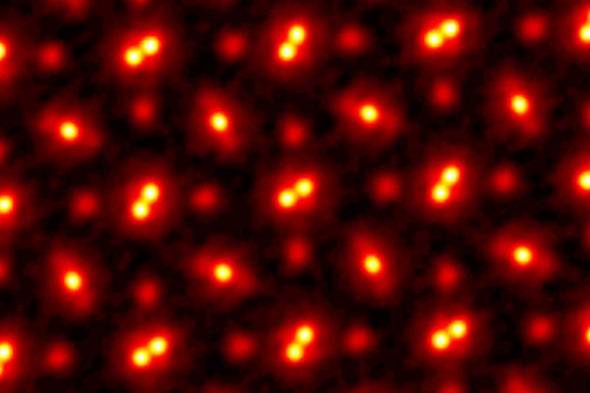
Image shows an electron ptychographic reconstruction of a praseodymium orthoscandate (PrScO3) crystal, zoomed in 100 million times. Credit: Cornell University
How does an atom really look?
In reality, we can't see an atom as we can see the collection of them. This is because the atom is almost 10000 times smaller than the wavelength of light. We are able to see a table because the light is reflected by it as an atom is very small the light will pass through the atom thus we cant see it.
We get answers by collision
Image No. 10324719 | This is a Rights Managed image. Inventory No.: 1901-51 Source No.: 1901-0051 Credit © Science Museum / Science & Society Picture Library -- All rights reserved.
The above image is a cathode ray tube. This is one of the original vacuum tubes used by the Cambridge professor of physics Joseph John Thomson (1856-1940) to discover the electron in 1897. Thomson's work contributed enormously to our understanding of the atomic structure of matter, leading to the research fields of atomic and nuclear physics, and marking the birth of the modern electronic age.
J J Thomson's scattering experiment gave a way to know and imagine the structure of an atom. You can get the exact detail on the internet. After the discovery of this experiment, the image of the atom was developed by many people and now we have a complex structure to understand but still, we can understand it.
The alpha particles were used in the early stages now we use the electrons, x-rays, and more to find out the structure.
Illustration of an atom
The is my imagination so if anything is wrong you can correct me.
Let's start with a primitive image and get an intuitive imagination.
Made by J John Paul using blender
CLASSICAL MODEL
The above is an image of an isolated Helium atom
(The colors are imaginary, they are provided to distinguish between the particles)
The above illustration gives us much information about the atom. On comparing the size of the fundamental particles we can see that the Protron and neutron are identical in size, So considering them as solids we can say that they have the same mass. By the experiments, it is found that the size of the Protron and neutron is 1.5 femtometer which is quadrillionth (1 followed by 15 zeros) of a meter or we can say 0.00000000000000015 meters. They both have almost the same mass. The mass of a proton is 1.67493 x 10^(-27) Kg and the mass neutron is 1.67262 x 10^(-27) which is very small. From the illustration, we can see that both proton and neutron collectively form the nucleus or the central part of the atom and they contribute almost 99.99% of the mass of the atom.
The electron is very tiny as compared to the above two particles. In terms of quantum mechanics, the electron is size less i.e. a point particle but it has a mass that is 9 x 10^(-31) m again it is very small.
Interestingly all the electrons in the universe are identical and you can't distinguish between two electrons if a cluster of electrons is placed before you. The same case applies to both protons and neutrons in the universe.
Made by J John Paul using 3-D Paint
MODERN MODEL
This is how a real scientist would imagine an atom. Previously we saw the two electrons in a definite position but in reality, we can't determine their exact position but we can predict their location in a specified region. Here come boring yet most beautiful reasoning of humans called probability. Probability is not a new word nowadays all the sports from football to cricket this plays an important role and we also involve in it like a modern astronomer.
So in the illustration, you can see some regions brighter than others. The bright region specifies the position of the electron with the maximum probability and the light region specifies the minimum probability of the position of an electron.
**** Now we have a good image of the electron and now we can proceed with some basic concepts.
New words to things before we dive into a beautiful scientific literature
- An isolated atom always consists of an equal number of protons, neutrons, and protons. It is because an isolated atom is natural so all net charges should be zero.
- Orbits - Orbits are the well-defined paths of the electron around the nucleus.
- Orbitals - The shape of the probability cloud is called orbitals.
Understanding orbits and orbitals
See the illustration of the classical model here we can see a very thin circular line that can be considered an orbit. It actually represents the motion of the electron in the 1-D plane. It can be either circular or elliptical. Orbits represent that position and momentum of an electron can be measured simultaneously with certainty.
Orbitals are 3- dimensional space (the real space we live in) around the nucleus where the probability of finding an electron is maximum. So it represents the motion of an electron in 3- dimensions. Orbitals have different shapes. Orbitals are completely a result of Quantum mechanics. So orbitals have many restrictions but it makes our job easy.
The main difference between orbits and orbitals is that in one orbit 2 x n x n electrons can be present (where n is principle quantum number) but in an orbital, only two electrons can be present that too with many restrictions.
To directly say orbitals are mathematical functions the describe wave-like behavior of an electron. The following illustration shows different types of orbitals. The orbitals are observed using spectroscopic techniques and are named after them according to their observed shape (s - sharp, p - principle, d - diffused).
ATOMIC ORBITALS
Illustration made using blender and GIMP by J John Paul
Instead, each electron exists as a probability cloud, more likely to be in one place than another, but not actually in any one place at any given time. The figures below show the various three-dimensional shapes of the probability clouds of electrons around a nucleus. The first type, called an “s” orbital, is totally symmetrical—the electron is not any more likely to be in one direction than another. The second type, called a “p” orbital, has two lobes, meaning the electron is more likely to be found on one side or the other of the nucleus, and less likely to be found in any direction in between. While there is only one “s”-type orbital, there are three “p” types, with lobes pointing in the three orthogonal directions (x, y, z) of space. Similarly, there are five different types of “d” orbitals and seven different types of “f” orbitals, with increasing numbers of lobes. (You may think of these shapes as a bit like three-dimensional standing waves.)
- Shells - The shell is the principle quantum number. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have different energies. Shells are named K, L, M, N.. (which are X-ray notations)
- Subshells - The "subshells" are the orientations and shapes for orbitals, going in order by s,p,d,f. (will be continued in quantum numbers)
- Energy levels - Everything in the world possess energy so when we enter into atomic levels we have to be careful with the energy levels. "A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels." In this case, we generally speak about the potential energy so by convention we set the potential energy at infinity is zero. Thus, the bounds electrons have negative potential energy. As the energy levels depend on the principle quantum number (n) these are not continuous rather discrete or quantized which means only a certain set of values can exist and others are forbidden. Quantized energy levels result from the wave behavior of particles, which gives a relationship between a particle's energy and its wavelength. For a confined particle such as an electron in an atom, the wave functions that have well-defined energies have the form of a standing wave (which is illustrated above).
- Ground state - When the electron is in the lowest possible energy configuration in a particular orbital it is said to be in its ground state.
- Exited state - If the electron is in a higher state than its ground state it is called an excited state. This may occur due to heat, light etc.
Rules for filling electrons in the orbits
- Aufbau's principle. It states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. Here the 1 in 1s represents the principle quantum number. Simply the electrons according to this rule are filled according to the increasing order of the atomic orbitals.
- Pauli's exclusion principle. This is one of the famous rule in the physics regime. To simply say it tells that in an isolated atom only two electrons can occupy the same orbital or the quantum state. This is because of the "spin" in electrons. Due to the spin no two electrons can take the same state. This rule help us to calculate the number of electrons that can occupy the given subshell ( it is given as 2(2l + 1) where l is the angular quantum number. It is named as exclusion principle because if two electrons with the same quantum state is present then one electron is excluded.
- Hund's rule. From previous rules we know that each orbital can occupy two electron. So while they are filled first one electron is filled first and then after fill it the 2nd electron is filled. i.e if you have the box and 6 balls first one ball is filled in each box and then the remaining 3 balls are filled in the box.
Filling of electrons in the atom
The four important quantum numbers
Quantum numbers describe the orientation of an atom mainly the location and arrangement. (previously you have encountered two quantum numbers n and l). It is easy to visualize an atom using quantum numbers because of the integer or half-integer values. Understanding quantum numbers will help us to know about the atom in detail.
- Principle quantum number (n): It is the serial numbers of shells starting from the inner lower most shells to the outer shells. Is has the values of 1,2,3,4... or generally represented as K, L, M, N.. shells. In a overall view it gives us the energy configuration of the atom. The set of orbitals with the same n value if often referred as an electron shell.
- Orbital quantum number (ℓ): It can also be called as angular quantum number because the orbital quantum number of an orbital determines its angular momentum an the shape of the orbitals (See the fig. of atomic orbitals above). It has the values (n-1) so it starts from 0,1,2,3... n-1. It is generally represented as s, p, d, f... which has the shapes spherical, sumblled, doughnut and some other unique shapes respectively. So it represents the number of planar nodes (mid point) passing through the nucleus.
- Magnetic orbital quantum number (mℓ): The magnetic quantum number specifies the orbitals available within a subshell, and is used to calculate the angular component of the orientation of the orbital in space when placed in an external magnetic field. So it wives two important things first, the projection of the orbital quantum number (ℓ) on the magnetic field direction. Its value renges from l, l-1, l-2 ...0, -1, -2, ... -l i.e. if l is 2 ml can have the values -2, -1, 0, 1, 2. This magnetic orbital quantum number forms the basis of the modern periodic table. s, p, d, f orbitals contains 1, 3, 5, 7 orbitals so the values of ml ranges from 0, +/-1, +/-2, +/-3. Each of these orbitals can accommodate up to two electrons with opposite spin. This quantum number brings the concept of spital quantization which means that if l=3 then ml can have 7 values thus the l or the angular component can take only 7 directions in the space.
- Magnetic spin quantum number (ms): As already mentioned an electron will have its own spin which is one of the fundamental property of electron. So an electron can have up (+1/2) SPIN or down (-1/2) SPIN, which gives the basic orientation of a electron. The ms give us the projection of the spin vector s along the direction of an external magnetic field. So it can have two values parallel to magnetic field (+1/2) and anti-parallel to the magnetic field (-1/2).
So with the above we can imagine a atom in more systematic and precise manner.
Electron configuration was first conceived under the Bohr model (classical model) of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.
An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. An atom's nth electron shell can accommodate 2 x n x n electrons, e.g. the first shell can accommodate 2 electrons, the second shell 8 electrons, the third shell 18 electrons and so on. The factor of two arises because the allowed states are doubled due to electron spin—each atomic orbital admits up to two otherwise identical electrons with opposite spin, one with a spin +1/2 (usually denoted by an up-arrow) and one with a spin −1/2 (with a down-arrow). A subshell is the set of states defined by a common azimuthal quantum number, ℓ, within a shell. The value of ℓ is in the range from 0 to n-1. The values ℓ = 0, 1, 2, 3 correspond to the s, p, d, and f labels, respectively. For example, the 3d subshell has n = 3 and ℓ = 2. The maximum number of electrons that can be placed in a subshell is given by 2(2ℓ+1). This gives two electrons in an s subshell, six electrons in a p subshell, ten electrons in a d subshell and fourteen electrons in an f subshell.
For atoms, the notation consists of a sequence of atomic subshell labels with the number of electrons assigned to each subshell placed as a superscript. For example, hydrogen has one electron in the s-orbital of the first shell, so its configuration is written 1s1. Lithium has two electrons in the 1s-subshell and one in the (higher-energy) 2s-subshell, so its configuration is written 1s2 2s1 (pronounced "one-s-two, two-s-one").
Representation of an atom.
HOPE THIS WAS USEFUL
TO SEE MY OLDER POSTS CLICK THE FOLLOWING LINK BELOW
Hope this article was useful and I hope you learnt something from it.
If you have any theories or questions regarding this you are free to express them in comments or you can chat with me in my Instagram page https://www.instagram.com/phy.sci/?hl=en.
For previous articles on previous series following link link